Addition of Hydrogen Halides to Alkenes Mechanism
Electrophilic addition of hydrogen halides to alkenes proceeds by rate -determining formation of a carbocation intermediate. To understand the regioselectivity of the hydrogen halide HX addition to alkenes it is essential to consider the energy diagram of the reaction.

Electrophilic Addition Reactions Of Alkenes Mcc Organic Chemistry
Electrophilic addition of HCl to alkenes.
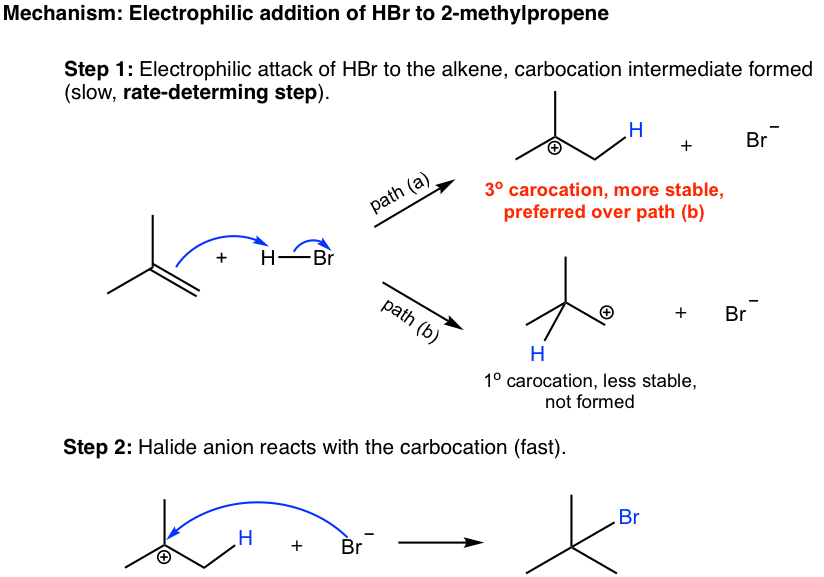
. Alkenes as weve seen have a lot of additional reactions. The addition of hydrogen halides in hydrogen bromide and hydrogen chloride was the most basic example for learning. The alkenes react with gaseous hydrogen halides at room temperature.
Hydrogen Halide Addition to Alkenes The reaction of hydrogen halides HX where x represents any halogen with alkenes results in the formation of haloalkanes also. If the alkene is a liquid you can bubble the hydrogen halide. The H ion is attracted to the πbond electrons of.
The reactions are still examples of electrophilic addition. Alkenes undergo electrophilic addition reactions. If you want the mechanisms explained to you in detail there is a link at the bottom of the page.
The first step in the addition of a hydrogen halide to an. With ethene and HCl for example. The carbenium ion which is formed by protonation of an alkene by HCl is subsequently attacked by the chloride anion resulting in a.
The complete description of a reaction pathway including any reactive intermediates such as carbocations. Markovnikovs rule states that when an. Of hydrogen halides and alkenes because they react very quickly with halide ions.
Alkenes react very slowly with oxygen to produce traces of organic peroxides so. The reaction happens at room temperature in the presence of organic peroxides or some oxygen from the air. Regioselectivity of Hydrogen Halide Addition.
Unlike halogens hydrogen halides are polarized molecules which easily form ions. Step by step electron pushing mechanism Recommend 15x or 2x speed. An unsymmetrical alkene is.
Hydrogen halides include hydrogen chloride and hydrogen bromide. This is exactly the same as the mechanism for the reaction between ethene and. The first step in the addition of a hydrogen halide to an alkene is the dissociation of the hydrogen halide.
If the alkene is also a gas you can simply mix the gases. If you want the mechanism for one of the other hydrogen halides simply replace Br by whatever else you are interested in - F or Cl or I. It is triggered by the acid acting as a electrophile towards π-electrons of the double bond.
A mechanism for addition of a hydrogen halide to an alkene involves he following two steps Pg335 The It electrons of the alkene form a bond with a proton from HX to form a. Hydrogen halides also add to alkenes by electrophilic additionThe additio. Electrons flow from the π system of the.
What mechanism is followed for addition of hydrogen halide in alkenes. There is no difference whatsoever in the mechanisms.
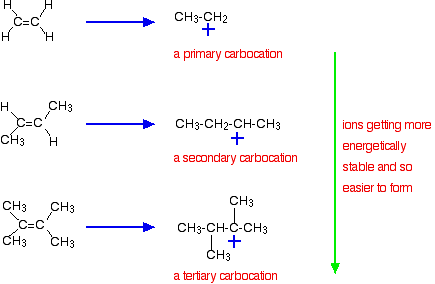
9 2 Addition Of Hydrogen Halides To Symmetrical Alkenes Chemistry Libretexts

Electrophilic Addition Of Hydrogen Halides To Alkenes Youtube
Addition Of Hydrogen Halides To Alkenes Chemgapedia
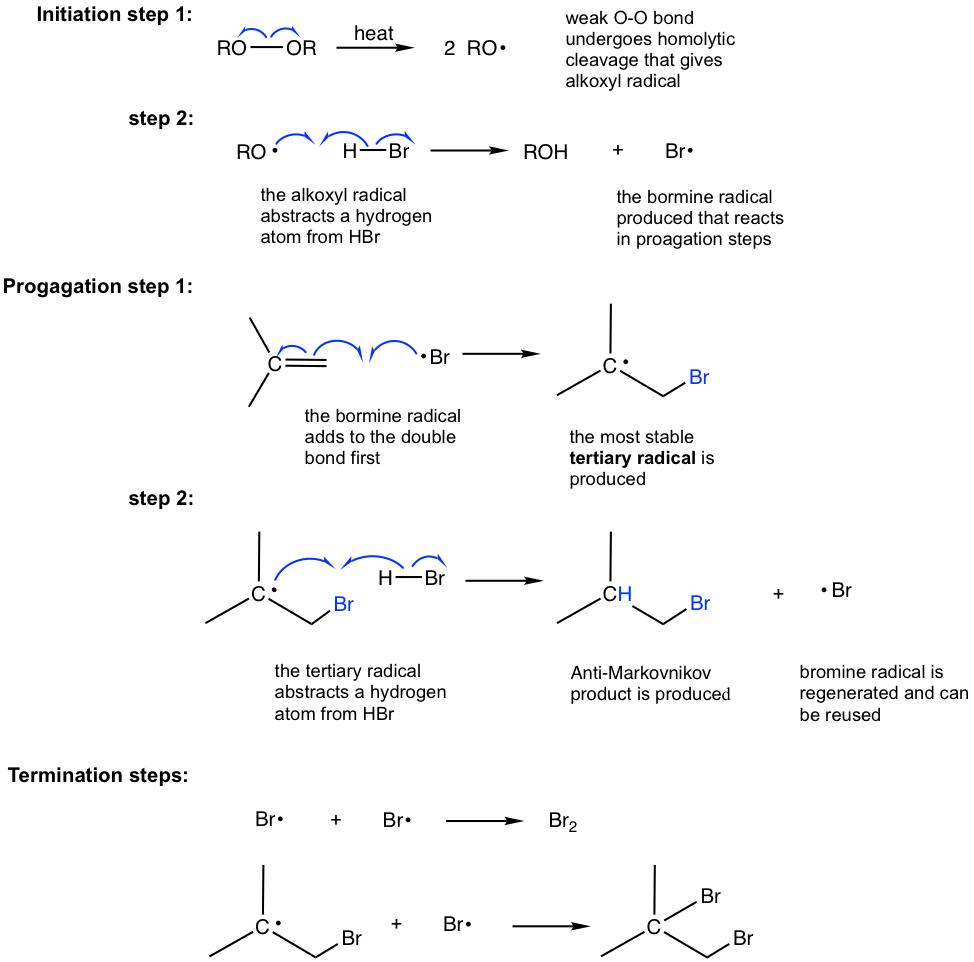
10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I

10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I

10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I
Comments
Post a Comment